How Long Does It Take For Water To Freeze? Time From Liquid To Solid
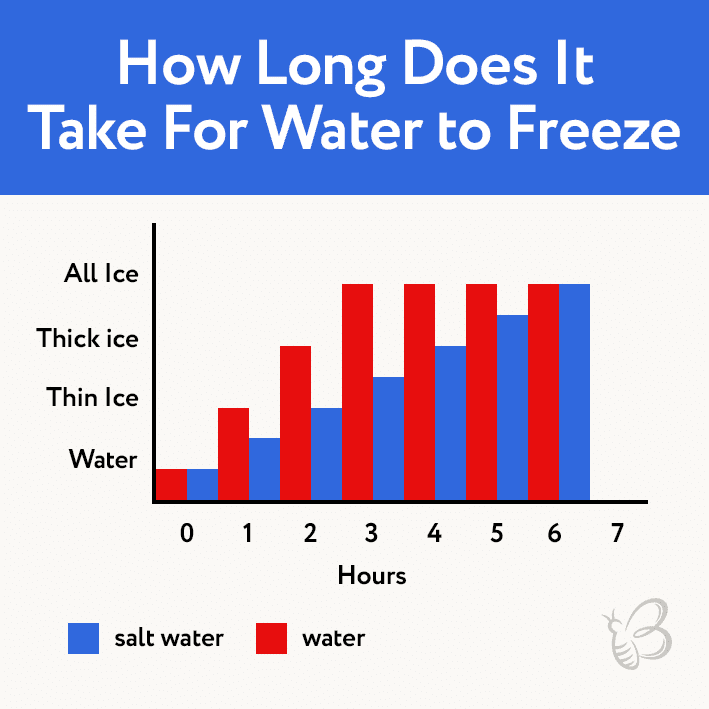
The time it takes for water to freeze depends on several factors, including the temperature of the water, the amount of water, and the presence of impurities. In general, the colder the water, the less time it will take to freeze. A small amount of water will also freeze more quickly than a large amount of water. Impurities, such as salt, can also slow down the freezing process.
The freezing point of water is 0 degrees Celsius (32 degrees Fahrenheit). However, water can remain liquid below its freezing point in a process called supercooling. Supercooled water is unstable and will freeze quickly if it is disturbed.
The process of freezing water is important for many natural and industrial processes. For example, the freezing of water in the atmosphere can lead to the formation of clouds and precipitation. The freezing of water in pipes can also cause them to burst.
- Premium Luxmovies Streaming The Best In Cinema
- Best Vegamovies Anime New Releases Popular Titles Your Ultimate Guide To Exciting Anime Adventures
How Much Time Does It Take for Water to Freeze?
The time it takes for water to freeze depends on a number of factors, including the temperature of the water, the amount of water, and the presence of impurities. Here are eight key aspects to consider:
- Temperature: The colder the water, the faster it will freeze.
- Volume: A small amount of water will freeze more quickly than a large amount of water.
- Impurities: Impurities, such as salt, can slow down the freezing process.
- Surface area: Water with a larger surface area will freeze more quickly than water with a smaller surface area.
- Agitation: Stirring or agitating water can speed up the freezing process.
- Pressure: Increasing the pressure on water can lower its freezing point.
- Nucleation: The presence of nucleation sites, such as dust particles or ice crystals, can speed up the freezing process.
- Supercooling: Water can remain liquid below its freezing point in a process called supercooling. Supercooled water is unstable and will freeze quickly if it is disturbed.
These factors are all interconnected and can affect the freezing time of water in different ways. For example, the presence of impurities can slow down the freezing process, but agitation can speed it up. The best way to determine how long it will take for water to freeze is to experiment with different conditions and observe the results.
1. Temperature
The temperature of water is one of the most important factors that affects how long it will take to freeze. The colder the water, the faster it will freeze. This is because the lower the temperature, the less energy the water molecules have. This makes it easier for them to form bonds with each other and create ice crystals.
- Exploring Wwwmasa49com A Journey Into Innovative Digital Solutions
- Hdhub4u Hindi Dubbed Your Ultimate Guide To Movies And Shows
- Facet 1: The freezing point of water
The freezing point of water is 0 degrees Celsius (32 degrees Fahrenheit). However, water can remain liquid below its freezing point in a process called supercooling. Supercooled water is unstable and will freeze quickly if it is disturbed.
- Facet 2: The rate of freezing
The rate of freezing is determined by the temperature of the water and the amount of heat that is removed from the water. The colder the water, the faster it will freeze. This is because the colder the water, the less energy the water molecules have and the easier it is for them to form bonds with each other and create ice crystals.
- Facet 3: The effect of impurities
Impurities in water can slow down the freezing process. This is because impurities interfere with the formation of ice crystals. As a result, it takes longer for the water to freeze.
- Facet 4: The effect of agitation
Agitation can speed up the freezing process. This is because agitation helps to break up the formation of ice crystals. As a result, it takes less time for the water to freeze.
These facets all contribute to our understanding of how temperature affects the freezing time of water. By understanding these factors, we can better predict how long it will take for water to freeze in different situations.
2. Volume
The volume of water is another important factor that affects how long it will take to freeze. A small amount of water will freeze more quickly than a large amount of water. This is because it takes less energy to remove heat from a small amount of water than it does from a large amount of water.
For example, if you put a small cup of water in the freezer, it will freeze more quickly than a large pot of water. This is because the small cup of water has less mass and therefore less heat to remove.
The volume of water is an important factor to consider when freezing water for any purpose. If you need to freeze water quickly, it is best to use a small container.
3. Impurities
The presence of impurities in water can significantly impact the time it takes for water to freeze. Impurities interfere with the formation of ice crystals, making it more difficult for water molecules to bond together and form a solid structure. As a result, the freezing process is slowed down.
One common example of this phenomenon is the use of salt to melt ice on roads during winter. Salt lowers the freezing point of water, preventing it from freezing at the usual temperature. This is because the salt ions interfere with the formation of ice crystals, making it more difficult for the water to freeze.
The effect of impurities on the freezing process is an important consideration in various practical applications. For instance, in the food industry, the presence of impurities in water can affect the freezing time of food products. This can impact the quality and shelf life of the food, as well as the efficiency of the freezing process.
Understanding the connection between impurities and the freezing process is crucial for optimizing various industrial and scientific processes that involve freezing. By controlling the presence and concentration of impurities, it is possible to manipulate the freezing time and achieve desired outcomes.
4. Surface Area
The surface area of water plays a significant role in determining how much time it takes for water to freeze. This is because the larger the surface area, the more water molecules are exposed to the cold air, which allows them to lose heat more quickly. As a result, water with a larger surface area will freeze more quickly than water with a smaller surface area.
This principle can be observed in everyday life. For example, a shallow dish of water will freeze more quickly than a deep pot of water. This is because the shallow dish has a larger surface area, which allows more water molecules to come into contact with the cold air.
The surface area of water is also an important factor in industrial and scientific applications. For instance, in the food industry, the surface area of food products can affect the freezing time. This is important for optimizing the freezing process and ensuring the quality of the food products.
Understanding the connection between surface area and freezing time is crucial for various applications involving freezing. By manipulating the surface area, it is possible to control the freezing process and achieve desired outcomes.
5. Agitation
The act of stirring or agitating water introduces small disturbances that disrupt the formation of a solid ice structure. As a result, this agitation provides more nucleation sites for ice crystals to form, accelerating the freezing process.
In practice, this principle can be observed in various real-life scenarios. For instance, churning ice cream involves constantly stirring the mixture to incorporate air and prevent the formation of large ice crystals. This agitation promotes the formation of smaller ice crystals, resulting in a smoother and creamier texture.
Understanding the connection between agitation and freezing time is crucial in numerous scientific and industrial applications. In the food industry, controlling agitation during the freezing process can significantly impact the quality and texture of frozen products. Similarly, in engineering, understanding the role of agitation in freezing can aid in designing efficient heat exchangers and cooling systems.
In summary, agitation serves as an essential factor in determining how much time it takes for water to freeze. By manipulating the level of agitation, we can control the freezing process, optimize industrial applications, and even enhance the quality of frozen products.
6. Pressure
The relationship between pressure and freezing point is an intriguing phenomenon with significant implications for understanding "how much time does it take for water to freeze;". As pressure increases, the freezing point of water decreases, meaning that water requires a lower temperature to freeze under higher pressure.
- Facet 1: The Role of Pressure in Phase Transitions
Pressure influences the phase transitions of substances, including the freezing of water. When pressure is applied to water, the molecules are forced closer together, making it more difficult for them to form the crystalline structure of ice. As a result, the freezing point is lowered.
- Facet 2: Implications for Natural Phenomena
This pressure-freezing point relationship has profound implications in nature. For instance, in deep ocean environments where water experiences immense pressure, the freezing point of water is significantly lower. This allows marine life to survive in cold, high-pressure conditions that would otherwise freeze water.
- Facet 3: Applications in Industry and Science
The effect of pressure on freezing point has practical applications in various fields. In the food industry, high-pressure processing is used to preserve food by inactivating microorganisms while maintaining the quality of the food. In cryobiology, controlled pressure is employed to preserve biological tissues and organs by preventing ice crystal formation.
- Facet 4: Relevance to Freezing Time
In the context of "how much time does it take for water to freeze;", understanding the pressure-freezing point relationship helps us comprehend the influence of pressure on the freezing process. Under higher pressure, water takes longer to freeze because it requires a lower temperature to reach its freezing point.
In summary, the connection between pressure and freezing point is an important factor to consider when examining "how much time does it take for water to freeze;". By understanding the role of pressure in phase transitions and its implications in natural phenomena, industrial applications, and freezing time, we gain a deeper insight into the behavior of water under varying conditions.
7. Nucleation
In the context of "how much time does it take for water to freeze;", understanding the role of nucleation sites is crucial. Nucleation sites, which can be dust particles or ice crystals already present in the water, provide a surface for water molecules to attach to and form ice crystals. Their presence accelerates the freezing process by reducing the energy barrier for ice formation.
- Facet 1: The Role of Nucleation Sites
Nucleation sites act as a template for ice crystals to form. In pure water without impurities, the formation of ice crystals requires a higher energy input to overcome the resistance to forming an ordered structure from the disorganized liquid state. However, the presence of nucleation sites provides a ready-made surface with a pre-existing crystalline structure, making it easier for water molecules to attach and form ice crystals.
- Facet 2: Real-Life Examples
In real-life scenarios, we can observe the effect of nucleation sites on freezing time. For instance, adding a small piece of ice to supercooled water (water below its freezing point that remains liquid due to the absence of nucleation sites) triggers rapid freezing as the ice crystal serves as a nucleation site for the surrounding water molecules.
- Facet 3: Implications for Freezing Time
The presence of nucleation sites directly influences the time it takes for water to freeze. With more nucleation sites available, the formation of ice crystals occurs more rapidly, leading to a shorter freezing time. Conversely, in the absence of nucleation sites, water can remain in a supercooled state for an extended period before freezing, significantly increasing the freezing time.
- Facet 4: Applications in Various Fields
Understanding nucleation and its impact on freezing time has practical applications in various fields. For instance, in cloud seeding, the introduction of nucleation sites (such as silver iodide particles) into clouds promotes ice crystal formation and precipitation, enhancing rainfall. Similarly, in cryopreservation, controlled nucleation is used to preserve biological tissues and organs by preventing uncontrolled ice crystal growth that can damage cells.
In conclusion, the presence of nucleation sites plays a significant role in determining "how much time does it take for water to freeze;". By providing a template for ice crystal formation, nucleation sites accelerate the freezing process, influencing freezing time in various natural and applied settings.
8. Supercooling
Supercooling is a phenomenon where water remains in a liquid state below its freezing point without turning into ice. This occurs due to the absence of nucleation sites, which are necessary for the formation of ice crystals.
- Title of Facet 1: The Metastable State of Supercooled Water
Supercooled water exists in a metastable state, meaning it is in an unstable equilibrium. Any disturbance, such as vibrations or the introduction of an ice crystal, can trigger the rapid crystallization of the entire water body.
- Title of Facet 2: Supercooling in Nature and Industry
Supercooling occurs naturally in various environments, including clouds and the polar regions. It also has industrial applications, such as in the production of glass and certain alloys, where controlled supercooling allows for the formation of unique structures and properties.
- Title of Facet 3: Implications for Freezing Time
Supercooling significantly impacts the time it takes for water to freeze. In the absence of disturbances, supercooled water can remain liquid for extended periods, delaying the onset of freezing. However, once disturbed, the water can freeze almost instantaneously.
- Title of Facet 4: Applications in Cryopreservation
Supercooling plays a crucial role in cryopreservation, the process of preserving biological materials at ultra-low temperatures. By supercooling cells and tissues, scientists can minimize ice crystal formation and damage, increasing the chances of successful cryopreservation.
In summary, supercooling is a fascinating phenomenon that challenges our understanding of the freezing process. Its unique properties and implications for freezing time have important applications in both natural and industrial settings.
FAQs on "How Much Time Does It Take for Water to Freeze;"
This section addresses common questions and misconceptions surrounding the freezing time of water, providing concise and informative answers to enhance your understanding.
Question 1: Why does the size of a water body affect its freezing time?
Answer: The surface area to volume ratio influences the freezing process. A larger surface area allows for faster heat transfer, leading to quicker freezing.
Question 2: How does stirring water impact its freezing time?
Answer: Stirring introduces disturbances that promote the formation of nucleation sites, accelerating the freezing process.
Question 3: Can water freeze instantaneously?
Answer: Under specific conditions, such as homogeneous nucleation or the presence of a strong electric field, water can undergo rapid freezing, appearing to freeze instantaneously.
Question 4: What is the significance of nucleation in water freezing?
Answer: Nucleation sites provide a template for ice crystal formation, significantly reducing the energy barrier and speeding up the freezing process.
Question 5: Can water remain liquid below its freezing point?
Answer: Yes, this phenomenon is known as supercooling. It occurs in the absence of nucleation sites or disturbances that trigger crystallization.
Question 6: How does pressure influence the freezing point of water?
Answer: Increased pressure lowers the freezing point of water, meaning it requires a lower temperature to freeze under pressure.
Summary: Understanding the factors that affect the freezing time of water is essential for various scientific and practical applications. By considering aspects such as volume, surface area, agitation, nucleation, and pressure, we can better predict and control the freezing process.
Transition to the next article section: This comprehensive analysis provides a deeper insight into the dynamics of water freezing, equipping you with a strong foundation for further exploration of this topic.
Tips on Understanding "How Much Time Does It Take for Water to Freeze;"
1
2
3
4
5
Conclusion
In this exploration of "how much time does it take for water to freeze;", we have examined the various factors that influence the freezing process, including temperature, volume, surface area, agitation, pressure, nucleation, and supercooling. Understanding these factors is crucial for predicting and controlling the freezing time of water in diverse scientific and practical applications.
The dynamics of water freezing hold significance in numerous fields, from cryopreservation and food preservation to cloud formation and engineering systems. By gaining a comprehensive understanding of the factors that affect freezing time, we can optimize processes, enhance product quality, and contribute to advancements in various disciplines.


Detail Author:
- Name : Carleton Rowe
- Username : crooks.leatha
- Email : orodriguez@dare.com
- Birthdate : 2006-12-19
- Address : 737 Drake Pine Mullerborough, MD 24798
- Phone : 941-243-9539
- Company : Bechtelar Inc
- Job : Social Scientists
- Bio : Nisi dolores iste aut rerum aspernatur facilis. Quo consequatur non temporibus dolor quis. Sit minima neque in autem et necessitatibus. Rerum odio voluptatum nemo sed nesciunt.
Socials
twitter:
- url : https://twitter.com/nelson.trantow
- username : nelson.trantow
- bio : Explicabo corporis voluptas qui tenetur. Ea molestiae temporibus totam incidunt ut doloribus mollitia. Quis at aliquam suscipit officiis autem quam molestiae.
- followers : 6404
- following : 2699
facebook:
- url : https://facebook.com/nelson_trantow
- username : nelson_trantow
- bio : Nesciunt commodi accusantium ipsam beatae.
- followers : 2589
- following : 2930